Get compliance and forecast metrics with our free disclosure dashboard

ClinicalTrials.gov and other registries are a treasure trove of clinical trials and compliance data. However, this valuable information is not easy to extract or analyze, even for sponsors who submitted the data in the first place.
RadarX provides a solution to this problem by automatically organizing information from ClinicalTrials.gov into a dynamic dashboard of metrics for analysis and compliance. It turns data into actionable insights.
Activity
View a history of your disclosure activities and metrics, even across multiple sponsor accounts.
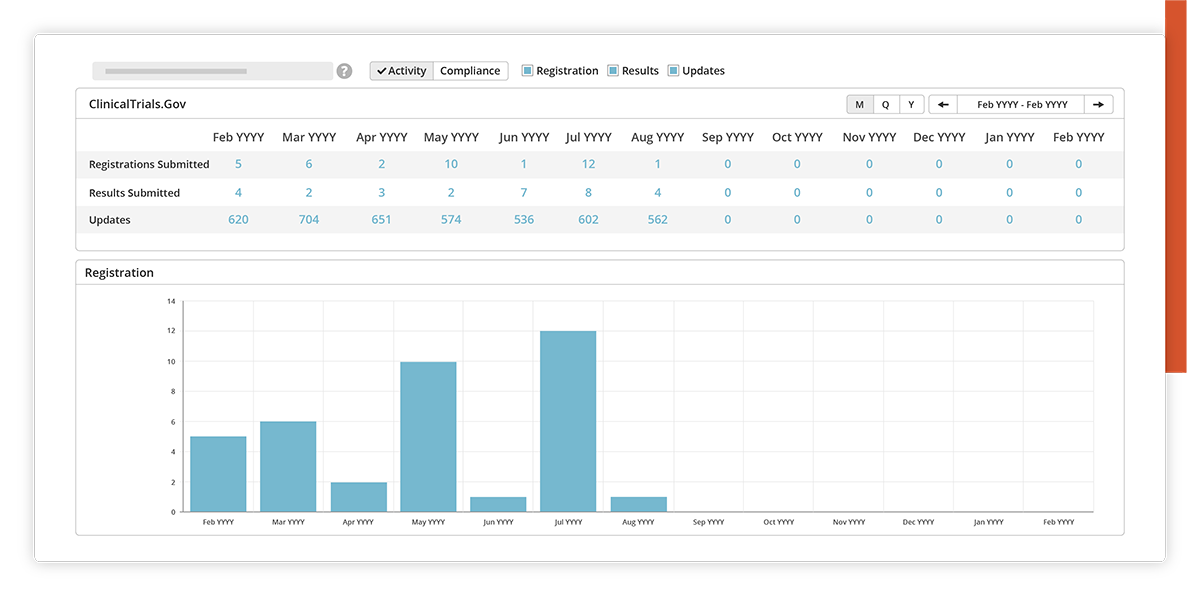
Activity
View a history of your disclosure activities and metrics, even across multiple sponsor accounts.

Activity
View a history of your disclosure activities and metrics, even across multiple sponsor accounts.

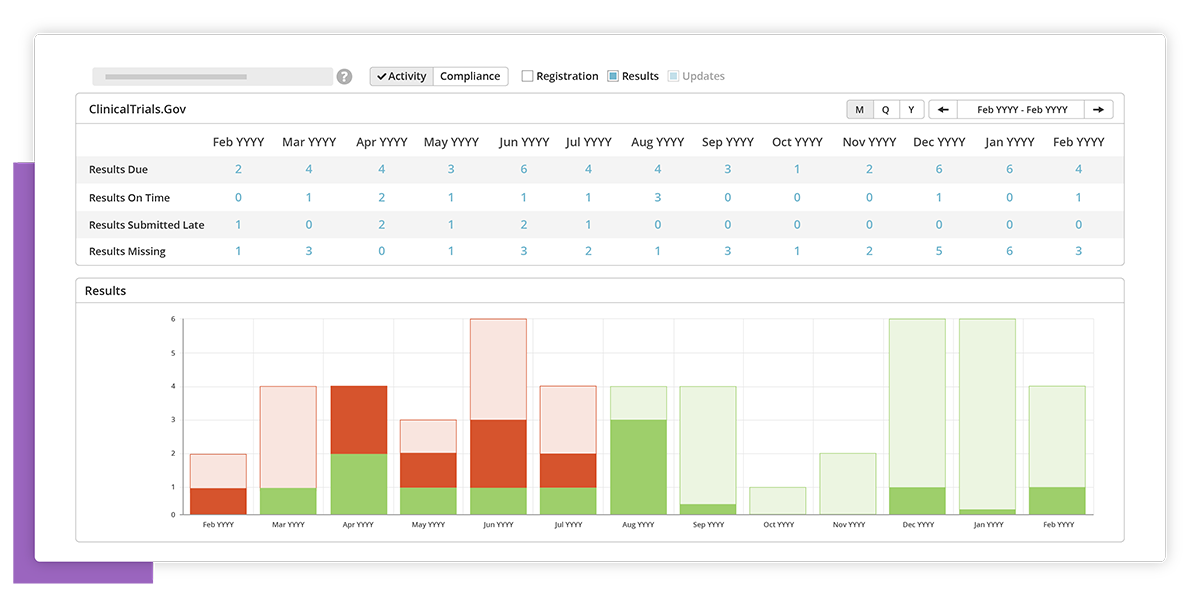
Compliance and forecast
See what studies are due for results disclosure and when. Forecast your activities and resource needs.
Compliance and forecast
See what studies are due for results disclosure and when. Forecast your activities and resource needs.

Compliance and forecast
See what studies are due for results disclosure and when. Forecast your activities and resource needs.

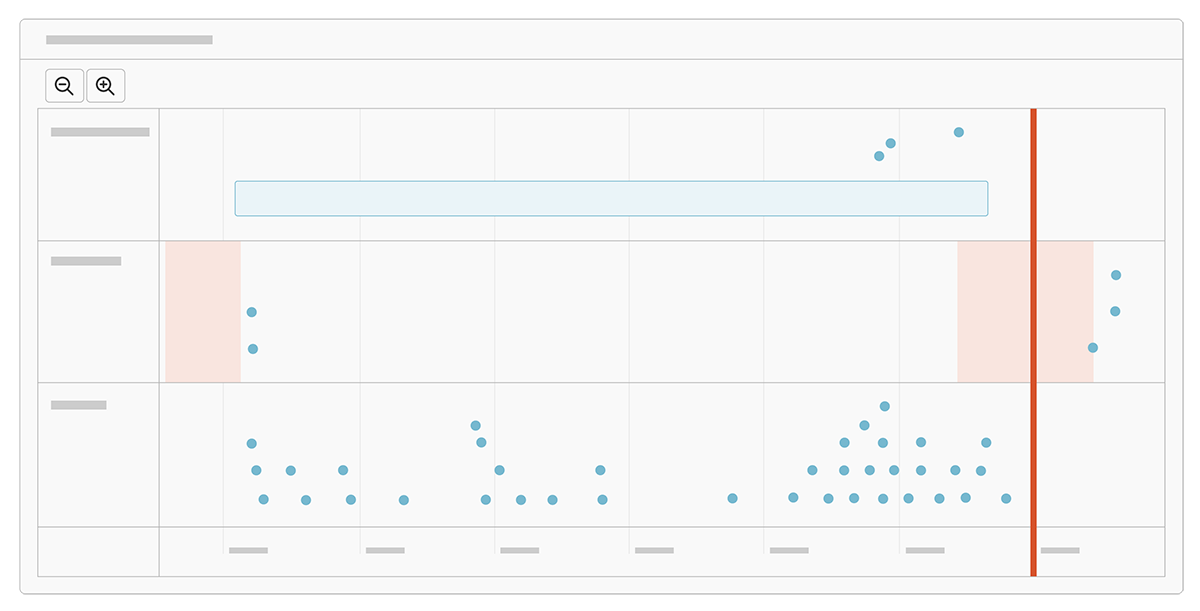
Drill down
Click and drill down to view the list of studies with compliance metrics.
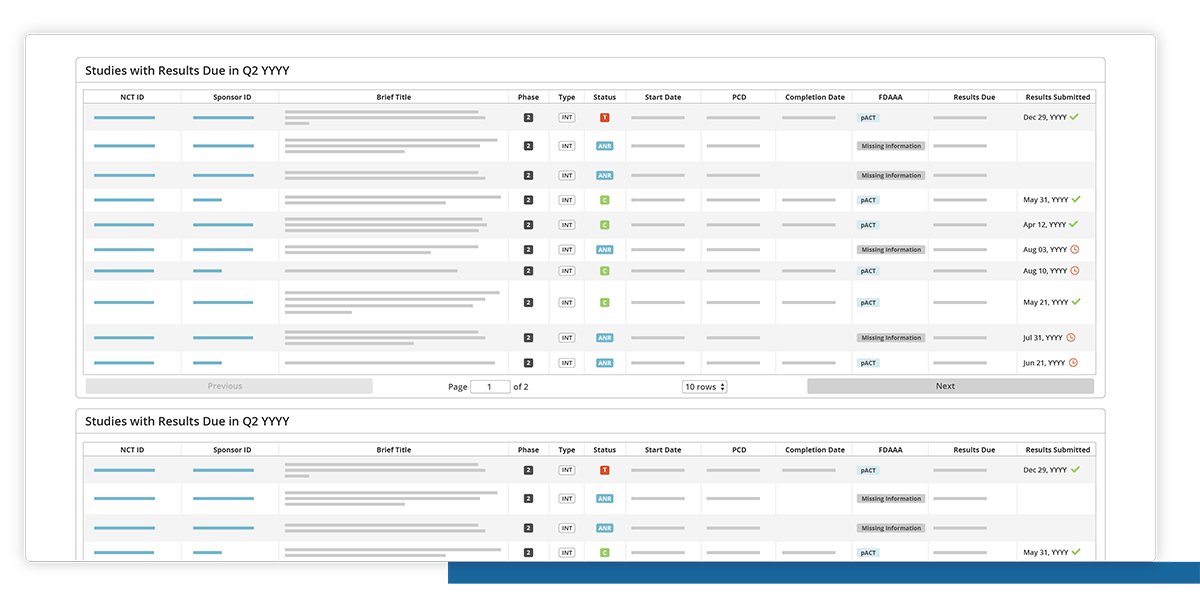
Drill down
Click and drill down to view the list of studies with compliance metrics.

Drill down
Click and drill down to view the list of studies with compliance metrics.

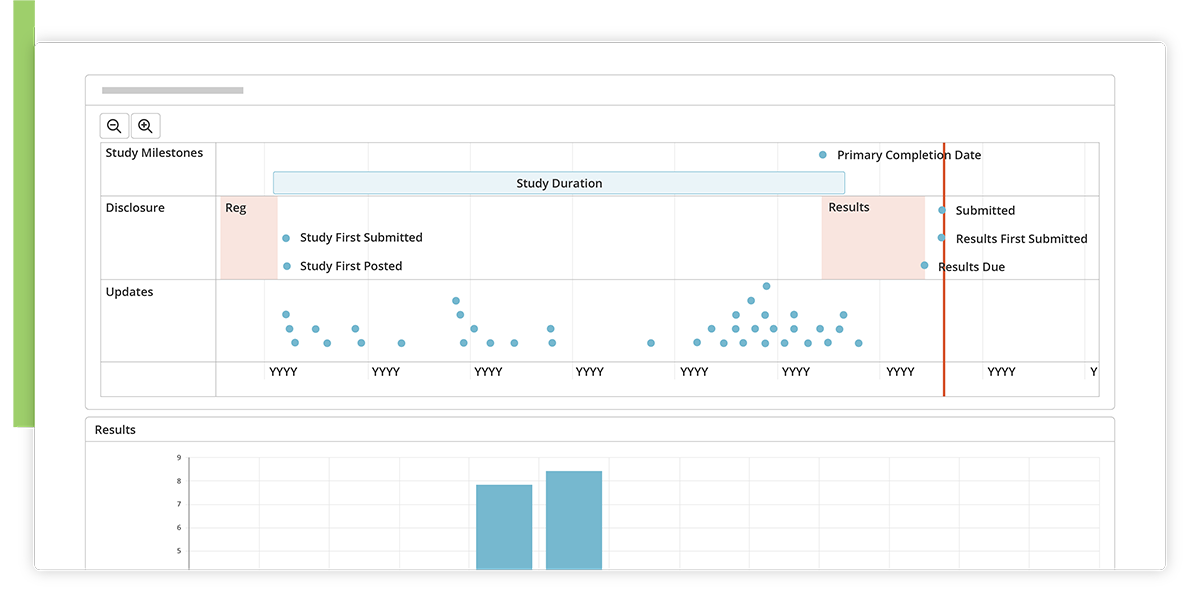
Timeline
See a visual timeline of all milestones and disclosure activities for a study.
Timeline
See a visual timeline of all milestones and disclosure activities for a study.

Timeline
See a visual timeline of all milestones and disclosure activities for a study.

Got questions?
We're here to help.
Got questions?
We're here to help.
By submitting this form, you agree to Xogene’s Terms of Service and Privacy Policy and also agree to receive emails from Xogene on educational resources, events, and product updates. You can unsubscribe at any time.
About Us
Careers
Solutions
Prime
Reach
RadarX
Services
Regulatory Intelligence
EU CTIS Submission Support
Advisory Services
©2024 Xogene LLC
Terms & Conditions
Privacy Policy
Terms & Conditions